Welcome to our Research Group web pages
The lab general theme:
Bacterial infections rely on the micro-organisms’ ability to adapt to its environment, subvert the immune system and multiply into its host. Our laboratory uses Structural Biology (Biochemistry, X-ray Crystallography, Cryo-electron microscopy and Biophysics techniques) a discipline that allows for a near atomic view of macromolecules to investigate how bacteria infect human tissues. We focus our investigations on particularly important ones: Helicobacter pylori, Brucella sp, or Francisella. These bacterial infections associates with various human diseases. Our goal is to provide the molecular details of key infection steps to drive new therapeutic strategies.
Helicobacter pylori type IV secretion system
Helicobacter pylori is a Gram-negative bacterium that colonizes the human stomach in half of the world’s population. Chronic infection by H. pylori is the main cause of gastric cancer, accounting for approximately 89% of distal gastric cancer cases. Gastric cancer prevention through eradication of risks factors, in particular H. pylori infection, seems to be the most promising way to control and eliminate this highly lethal disease. However, not all strains of H. pylori are detrimental to human. Strains carrying the cag-pathogenicity island (cagPAI) are more frequently associated with severe diseases. This cagPAI is a 40 kbp DNA region that encodes for a type IV secretion system (cagT4SS) and for the CagA oncoprotein. Upon contact with gastric cells, the cagT4SS delivers CagA into epithelial gastric cells. Once injected, CagA attaches to the inner leaflet of the membrane of the cell where it can be phosphorylated by host cell kinases and interacts with a plethora of cell signalling proteins, hereby promoting tumours development.
Ou group aims at understanding how the cagT4SS delivers CagA at the molecular level. We collaborate with Dr. Rémi Fronzes (IECB, Bordeaux), Prof. Wolfgang Fischer (LMU, Munich, Germany) and Dr. Patricia Rousselle (LBTI, Lyon).
Brucella Type IV secretion systems and effectors
Brucellosis is one of the most prevalent zoonotic diseases, with around 500,000 new cases reported each year in the world, predominantly in developing countries, causing mayor economical as well as human health problems. The disease is caused by bacteria of the genera Brucella spp., facultative intracellular pathogens with the capacity to infect a broad range of cell types, tissues and organisms. Brucella deploys a set of virulence factors that manipulate the host cell in its own benefit to establish a safe intracellular replication niche. These virulence factors, generically called effectors, are proteins delivered by the bacterium to either the cytoplasm of the cell or the BCVs, that interact with specific proteins of the host, modifying their biochemical activities in benefit of the pathogen. These effectors can be secreted and/or translocated by a type IV secretion system (T4SS), a “molecular syringe” produced by the bacteria.
Work on Brucella effectors and T4SS is performed in collaboration with Pr. Suzana Salcedo (University of Madison, USA), Prof. Jean Celli (University of Vermont, USA), Dr. Juan Ugalde and Dr. Diego Comerci (University of San Martin, Argentina) and Dr. Cascales (LISM, Marseille).
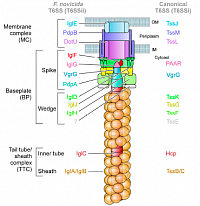
Type IV secretion system (T6SS)
Type VI secretion systems (T6SS) are molecular nanomachines that ressembles a crossbow . They are widespread in bacteria and are used to translocate effectors in other bacteria and kill them of in human cells to promote infection.
We study the T6SSs and their effectors in Escherichia coli in collaboration with Dr. Eric Cascales (LISM, Marseille) and in Francisella tularensis in collaboration with Dr. Thomas Henry (CIRI, Lyon)
Other projects
We are studying also some individual effector proteins from various secretion systems and bacterial pathogens.